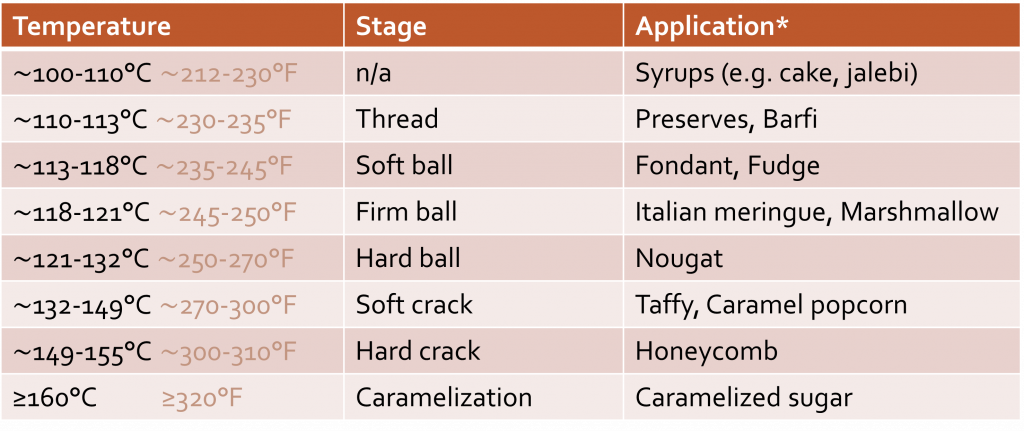
Textures & Stages of sugar syrups
Did you notice how the consistency of a sugar syrup can chance based on the temperature it’s been boiled to? Keep in mind that these sugar solutions are made of just sugar and water! We didn’t add anything. Pretty amazing how sugar & water interact isn’t it?
Did you notice that the syrup got thicker and harder at higher temperatures? This is because there is less and less water. Water facilitates free movement of all the molecules. When there’s more sugar and less water, movement becomes more strained. The sugar molecules bind part of the water and they like to interact with one another.
You can probably also imagine how you’d boil sugar to a different temperature depending on the final application. If you’re looking for a hard, brittle texture, you’ll boil it to a higher temperature, than let’s say when you make a soft caramel! Below you can find some examples of what temperatures cooked to different temperatures might be used for.
Keep in mind that other ingredients, including other sugars, will impact your final texture again. So even though this temperature is a great starting point, there are more ways to influence a candy’s texture than by just changing the temperature to which it’s boiled.
Remember the state diagram?
Remember the sucrose state diagram? The boiling temperatures mentioned below would lie on the orange curve, the boiling point elevation. At high temperatures, the sugar solution only contain a very (very) small amount of water. Once sugar starts to caramelize just about all water has gone. In the stages before that, the water content is also quite low already.
Ice-water test
We have known this relationship between temperature and sugar concentration for a long time. Even before most people had access to proper thermometers in their kitchen, they could use this system. Instead of using a thermometer, they would take a little bit of the sugar syrup and cool it down very quickly. By doing so, you ‘freeze’ the system. This way, you get an idea of how the syrup will behave once it’s cooled down. Will it be soft, or hard for instance?
This is the so-called ‘ice water test’ since you would drop the sugar syrup in a bath of ice water. Whereas it’s a good test, using a thermometer does make things easier when you make the same candy a few times.
Temperature increases faster and faster
When you’re boiling a sugar syrup, chances are you felt like the first 10 degrees (e.g. from 100 to 110°C) took ages, whereas the last 10 degrees (e.g. from 130 to 140°C) went by very quickly.
That’s not just a feeling. That’s actually true.
In the beginning, a lot of moisture needs to be boiled off. However, the warmer the syrup becomes the less water needs to evaporate before the temperature can increase with yet another degree. So, yes, whereas in the beginning you might be able to quickly walk away for a few seconds, you definitely can’t do that towards the end.
This is all sucrose
Keep in mind that all of this applies to sucrose (regular sugar) solutions. When you start adding other sugars, the consistencies and textures can change quite dramatically.
